Storage batteries are the “gas tank” of any off grid power system. When the sun isn’t shining or the wind isn’t blowing, batteries make up for the deficit. The only real alternative to storage batteries is a mechanical generator, which of course needs fuel. Since constantly running a generator isn’t practical or affordable for most of us, batteries are the default choice. Storage batteries are often one of the most neglected parts of an off grid power system; we’re going to walk through the basics and learn how to get the most out of our investment.
What exactly is is a storage battery?
Technically, all batteries are “storage batteries”. For our purposes, a storage battery is a large battery or bank of large batteries that are used as a long term general power source for a variety of devices, lights, and appliances. This is not the same as small batteries intended to power a specific device, such as a handheld radio.
Electricity is an on-demand service, meaning, your power company does not make any electricity until the very moment it is required. Because alternative energy sources (solar, wind, etc) are erratic and no one can exactly predict when they will be available, it’s nearly impossible to synchronize demand with supply. This dilemma has frustrated engineers for decades and is the main reason why renewable energy is not widely used for commercial power generation.
The radio amateur has an advantage that the big power companies do not: Storage batteries. Storage batteries are affordable and fairly easy to deploy on a small scale. They allow radio amateurs to store energy when it is available for use at a later time. They are what makes off grid ham radio possible.
Location, location, location.
Where storage batteries are kept will have an effect on their service life and performance. An indoor, climate-controlled location is ideal. If that’s not possible (and for many of is its not), a “semi-climate-controlled” place is acceptable. “Semi-climate-controlled” is an area that may not be directly heated or cooled but is a somewhat stable, indoor environment, such as an attached garage or basement. The goal is to keep storage batteries between 35-100 degrees. Fortunately that range of acceptability is doable for most hams.
Example: My storage batteries are in a utility closet that has two exterior walls and no air vents connected to my heating/AC system. I consider that semi-climate-controlled. Where I live in the upper Midwest USA we can go from well below zero in January to the 90s and above in August. Through all the seasons the temperature in the closet ranges from the mid 50’s to about 80º F. And by the way, yes, you absolutely should monitor the temperature and humidity in your storage battery area.
Storage batteries do not like excessively high or low temperatures, and the less humidity the better. If you must keep your batteries in a compromised location such as a shed or detached garage, then carefully following the maintenance procedures in this article will help you get the most out of your investment. Even then, you will have to accept lower performance and shorter service life.
Also keep in mind that in a long term grid down scenario, artificial climate control as we know it is not going to be there. Always plan for the worst.
Many MPPT charge controllers have a temperature sensor that goes on one of the battery terminals. This sensor allows the controller to make small tweaks in the charge voltage according to the battery temperature automatically. Use of this option is highly recommended and helps compensate for the effects of temperature swings.
Storage Batteries Tools Of The Trade.
Maintaining your storage batteries is not particularly difficult, but you will need some tools. Luckily, all but two are inexpensive and you probably already own them. The ones that are expensive can be omitted if the budget is tight. Most of these tools are straightforward. A few require a little explanation:
- Socket wrench & set.
- “Z87” Safety glasses with side shields (ANSI Z87.1 compliant)
- tight fitting work gloves
- small wire brush
- screwdriver
- pliers
- emergency eyewash and first aid supplies
- battery brush: A round wire brush designed specifically for round battery terminals; available at any auto parts store.
- anti-corrosion compound: A product applied to battery terminals to prevent corrosion. Comes in paste or aerosol; either form is acceptable. Available at any auto parts store.
- For wet cell/flooded batteries only: hydrometer and distilled water. A hydrometer is a very simple and inexpensive (less than $10) calibrated device also available at any auto parts store that measures the density of the electrolyte in wet cell batteries. The acid density is also an indicator of the state of charge of the battery.
- battery load tester (optional): I use a fixed 50 amp model that I bought new for $25 a few years ago but when I researched them for this article I could not find a 50 amp version anywhere. All the inexpensive load testers currently for sale are 100 amps, which I think is too high for the average off grid ham’s storage batteries. The only alternative is the much more expensive variable carbon pile load testers which allow the user to set the load from zero to several hundred amps.
- digital battery analyzer (optional): These gizmos can range in price from $50 all the way up to over $1000. Mine is a Centech (Harbor Freight) that cost about $80 and performs well above its price tag. Some digital analyzers have a load test function built into them, so if you select one of these models, you don’t need a separate battery load tester.
In addition to the above you will want to keep a stock of post clamps, connectors, and any other hardware unique to your storage batteries.
I suggest that these tools and supplies be kept in a separate box dedicated solely to battery servicing. You do not need to have high end hand tools because they will only be used a few times a year for storage batteries. Except for the test devices, you should be able to assemble your entire kit for less than $50.00.
Procedure for testing and maintaining your storage batteries.
There are two main types of storage batteries used by off grid hams: Flooded or wet cell batteries, and sealed batteries. Sealed batteries have several sub categories. The maintenance procedures for both flooded and sealed are the basically the same with the exception of checking & filling electrolyte levels on flooded/wet batteries, which we’ll go over. This process assumes you are starting with fully charged 12 volt batteries.
Wear tight fitting work gloves and eye protection.
1. Disconnect the storage batteries from the load by removing the fuse or operating the cutoff switch. If there is no fuse or switch (why???) then disconnect the leads. As we have discussed in previous Off Grid Ham articles, remove the negative lead first.
2. Disconnect the individual leads from only one battery in the string (again, negative first). Using the wire brushes, thoroughly clean both the clamps and the battery posts. Where the wiring attaches to the clamp, carefully inspect for for corrosion or loose connections. Wipe off any dirt or debris. Inspect the battery case for cracks, bulges, or leaks. If any are found, the battery cannot be saved and must be replaced.
3. (For flooded/wet batteries, skip to step 5, then come back here) Leave the battery sit idle with no charge source or load for at least fifteen minutes, then measure the voltage. You should see a minimum of 12.65 volts on an unloaded, fully charged 12 volt battery. If you do not, connect the battery to a conventionally powered plug in charger until the charger completes its cycle or the charge current is very close to zero. Remove the charger, let the battery idle another fifteen minutes, and measure the voltage again. If the voltage has bounced up to 12.65 or higher, then the battery is good and there is a system malfunction not inherent to the battery itself. Check the connections, the output of the charge controller, etc. If after the charge cycle the voltage is still too low, then the battery probably needs to be replaced.
4. Treat both the battery terminal post and the clamp with anti-corrosion compound. You do not need to goop it on heavy; a light coat will do. Reconnect the leads, positive side first. Make sure the terminations are clean and tight.
Repeat steps 1-4 for each battery in your string.
5. Flooded/wet cell batteries only: Before starting this procedure, it is absolutely imperative that you wear proper gloves and eye protection that includes side shields. Also, keep a bottle of emergency eyewash and appropriate first aid supplies nearby. I’m not deliberately trying to be dramatic or freak anyone out, but it must be completely understood that one tiny drop of battery acid flung into your eye can have irreversible lifetime consequences, and contact lens wearers are at an even higher risk of permanent injury. Sulfuric acid is extremely dangerous but the protective measures are simple and effective. Also, wear crappy clothes. Any acid that gets on your clothes will eat a hole in the fabric. I don’t care how careful you think you are, you will get that stuff on you. The damage will not appear right away so for a while you’ll think all is ok. If you get any on your bare skin, rinse well with water. Keep a supply of baking soda in your battery area to neutralize any acid spills.
DO NOT: Smoke, work near open flames, sparks, or ignition sources when performing these tasks. Wet/flooded batteries emit hydrogen gas, which is very unstable and highly explosive. Make sure your work area is well ventilated.
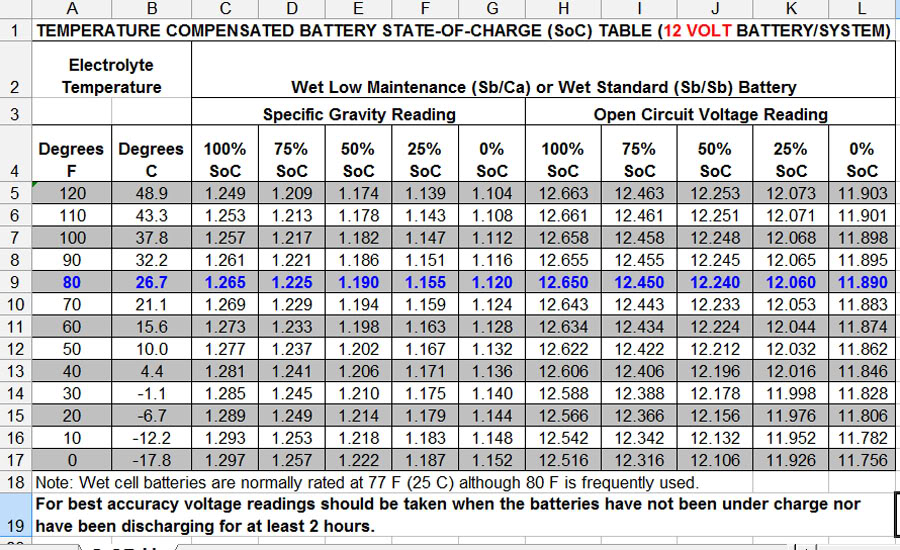
Wipe any dirt or debris from the battery. Pry open the cell covers. Using your hydrometer, test each individual cell. At 80º F the density should be no less than 1.225 in each cell (a higher number indicates a higher charge state), and each cell should have approximately the same density. Temperature is a factor when measuring acid density. Refer to the chart below to determine acceptable readings for your ambient temperature. If any cell or cells are below 1.225, the entire battery may need to be replaced, but don’t give up yet. There is a possible fix.
Using a flashlight, look down into each cell. The electrolyte level should at least be above the plates. Exposed plates suggest a leaking or neglected battery. Fill each cell with distilled water to just below the fill hole. Do not use tap water, bottled drinking water, or nursery water. Distilled water only! You can distill your own water if you really want to rough it, but that’s more trouble than it’s worth. Go easy on yourself and buy distilled water in any supermarket for less than $1.00/gallon. For disaster preparation purposes, keep at minimum one gallon for every two storage batteries in your setup.
When you are done with step 5 and the filler plugs are firmly secured, wipe off the batteries with a damp cloth. Rinse the inside and outside of the hydrometer with plain water. Also wipe off tools or anything that may have come in contact with the acid. Finally, wash any exposed skin. You should not need to wash your hands because you were wearing gloves, right?
Tech tip to avoid a rookie mistake: If you measure the voltage or acid density immediately after topping off the water, it is possible both metrics may read low because you just added straight water to the cells and temporarily diluted the sulphuric acid, forcing the battery into a discharged state. If this happens, place the battery on a charge source for a full cycle. Then go back to step 3. Follow the instructions from there.
Here’s that possible fix for single cell or cells with low density: After completing steps 3 and 5, measure the density in the cell(s) that were low. It will hopefully now be at 1.225 or higher. If it is, then you have successfully “rescued” your battery! If the density is still below 1.225 but the overall battery voltage is at least 12.65, then your battery is useable for now but be warned it is on the decline and will need to be replaced soon. A voltage below 12.65 after a full charge cycle indicates a weak/dead battery no matter what the cause.
Analysis and load testing of storage batteries:
There are two approaches to battery testing: To check each battery individually, and testing the entire string as a whole.
Digital battery analyzers usually require the user to manually enter the capacity of the battery. If you are testing the string as a whole, make sure you add up the sum of all the batteries and enter that as your capacity or the result will be inaccurate.
For load testing, as long as the batteries checked out ok in the previous steps, I see no need to load test them individually. I connect my load tester to the main buss coming out of the string and monitor the starting, unloaded voltage. I then induce a 50 amp load for 5-7 seconds. A good string will show a voltage drop to no less than 11.4 volts. When the load is removed, the voltage should recover to very close to what it was at the beginning of the test.
Keep in mind that the 50 amps used for testing my storage batteries is based on my 515 amp-hour total capacity. If you have a lot more (or less) capacity, 50 amps my be too light or too heavy a load. I suggest a amperage load of about 10% of total capacity, so if for example you have 700 amp-hours of battery capacity, ideally you would use a 70 amp load for your test.
How often should you perform these steps on your storage batteries?
Perform these routines every six months for storage batteries in a controlled or semi-climate-controlled environment. Batteries kept in harsh environments or exposed to extreme temperatures or humidity should be serviced more often, perhaps quarterly.
What if I’m using different voltage batteries?
The procedures explained here assumes 12 volt batteries. If your batteries are some other voltage, the basic steps are the same. The only difference is the test voltages and current will be need to be adjusted for your application and you may have difficulty finding analyzers and load testers for nonstandard voltages.
If a battery has failed…there’s bad news on top of bad news.
If for any reason a single battery in a common group is not passing tests and is determined to be at the end of its service life, you’re in a bit of a bind because you cannot simply remove the dud, replace it, and leave the others as they were. Batteries wired in a common string have to be placed into and removed from service as a set. This can really suck because if for example you have a string of four storage batteries but only one of them is bad, you have to pay for four new batteries when you really only need one. There is no way around this. Mixing old and new batteries will drag all of them down.
You can repurpose the remaining good batteries on other projects, or keep them in your existing system by connecting them through a battery combiner. This early Off Grid Ham article discusses what battery combiners do and how to use them.
Storage batteries are a consumable item and the weak point in any off gird energy system. They are expensive and difficult to replace, so it is worthwhile to spend the needed time to protect your investment. The steps outlined here are not as tedious as they appear. Regularly servicing your storage batteries will let you get the longest life and best performance from them as well as serve as an early warning of problems.
Batteries are a pain! But what are ya gonna do? Got to have them. Can’t do business w/o them. Great post!
Yes,batteries probably cause the most hassle in any off grid system. The only other option is a generator, which over long term use is more of a pain than the batteries. 73, Thanks for stopping by!
Es el gran problema del radioaficionado cuando está fuera del QTH, alimentar su equipo para poder comunicarse, hay baterias infinidades de modelos y tipos, el handica es el peso, poco pesada menos tiempo de transmisión, muy pesada no soportaremos el peso, nos quita las ganas de salir, en fin peso o no peso he ahí la cuestión, 73, saludos
Saludos, Vando y gracias por la lectura de la Off Grid Ham. Este artículo fue fijado sobre todo acerca de las baterías de almacenamiento donde el tamaño y peso no son mucho de un tema. Usted tiene un punto que los ordenadores se utilizan a menudo para los modos de QRP, y que puede ser un problema cuando se opera como una estación portátil. En un próximo artículo voy a hablar de pequeñas baterías y cómo ejecutar QRP portátil effectve. Gracias y por favor, ven por fuera Off Grid Ham pronto.
Ahh….well, I should probably write a book on flooded 6v golf cart battery arrays. For any household sized array wash it down with water…….yup, that means one has to have a box, collection buckets, etc but at least one will have pressurized water to clean your body, especially your eyes. Gases will be vented, some are explosive, and eventually an arc will ignite them. Big boom or little ‘pop’ will depend on how aware you are to the potential. Keep lead acids in a different building than nickel-irons/other alkalines. Shield inverters, etc from acid. It spatters, flies around, when serious charging is going on. When flooded golf batteries get old you must check each individually and the older they get, the more often, however this also means you can mix great and fair batteries depending on how much followup you want to do. One will waste a lot of money (~$100 each) for every mis-diagnosed battery. Once or twice a week might be needed to avoid dry-cell arcing. etc. Handheld infrared sensor can find problems earlier. A wrench in hand can nearly vaporize if dropped or mis-handled.
Forgot to mention that I’ve found generic golf cart batteries life to roughly last 3 years under rough use. They’ll hiss, pop, and leak acid up the posts and stink up the battery room. Run ’em till they drop!
When flooded lead acids get ‘touchy’ is when they age, have dissimilar acid concentrations, different sulfation rates, high battery or battery room temperatures, and/or poor terminal connections. This is why Chris says, “replace the whole bank”. Consider a cell in an array that charges really fast. This also means It has little capacity. Now intersperse several poor cells in an array. You’ll note some lack of capacity but until you try to pull 100 amps or more out of the bank the excessive voltage drop might be missed. What comes next is a possible battery terminal meltdown. Another possibility is the boiling of weak acid/mostly water until the electrolyte level gets too low and an arc ignites gas exploding the case. Having a poor connection might mean a hot terminal or the accumulation of low performing cells is concentrating amperage drain to only one part of the array. Hot terminals can result from acid soaked corrosion and the heat may loosen the nut as the bolt terminal is cast into the lead and changing temperatures affects tightness. Spraying water on corroded terminals can boil to show a bad connection. Getting used to these possibilities means your arrays condition/life can be determined and possibly extended with the help of distilled water, sulfuric acid, hydrometers, thermometers, load testers and computer load analysis. Many times batteries are mis-labeled for scrap when other variables were the cause. Check them three times under different conditions, usually during the equinoxes when you might have extra power.
Hi Jack,
As mentioned in my article, a hydrometer is very valuable and is the only practical way to know the status of an individual cell. Your comments on battery terminals is well taken, but I think you will know you have a problem with a battery long before the terminals get hot enough to boil water! Speaking of battery terminals, it is important to make sure they are clean and tight, but not over tight. In my “real job” I work with wet cell batteries that are half the size of a refrigerator, and the manufacturer specifies that the terminals must be tightened to a specific torque specification. Too loose, and you create a resistance that generates heat as you describe. Too tight and the soft metal connections can crush or crack (sometimes internally where the damage cannot be seen) and likewise create a resistance. And trust me…when one of those big boys lights up, you have one hell of a problem! Batteries that hams use are not as fussy, but you do have a good point.
Thanks for your input Jack. Hope you’ll come back to Off Grid Ham soon.
Had two “boiling” terminals today in a 20 6v golfcart battery array. Don’t know what causes them. The problem with hydrometers is knowing the current state of the array and the condition of the individual battery in the array which is varying while in use. In an aging array dissimilarities must be treated and eventually removed. So, the rich guy can replace an array without the bother and the poor guy can limp along for maybe another year or so with daily to weekly attention to every cell in the array. Cells do not charge evenly so I call the poor mans servicing ‘cell balancing’. Position in the array, temperature compensated hydrometer readings, load tester coil temperature, past individual cell performance, gassing tendency, plate color, electrolyte color, electrolyte on top of batteries, and battery temperature all matter. Larger batteries, maybe like Chris works with, can be more stable and larger arrays of smaller batteries are wonderful when new but the servicing of flooded lead acid aging arrays is a challenge and an art. Still learning 🙂
Hi Jack. You are exactly right…there is a bit of art or as I like to say, finesse to dealing with flooded batteries. You’ll never have everything perfectly balanced and in alignment, especially if you are using consumer-grade batteries. The large arrays I work with professionally are 2.20 volt (or so) cells wired together in groups of 24. One of the the benefits of this system is that cells can be much more closely monitored and tested. The bad news is that such a setup is extremely expensive and labor intensive.
Thanks for your input. I hope you’ll come by Off Grid Ham again soon.
So, I’ve found it best to have 2 arrays of flooded lead acids. The primary array is of new and cells/batteries checking out in the green on a load tester and showing good electrolyte specific gravity throughout. The secondary bank are the others, have individual cell electrolyte SG differences, exhibit acid climbing the terminals or are bubbling through the caps, have higher charge temperatures, bulging cases and melted terminals. 100 amp alligator clamps (Mueller BU-21cp?) on 24″ heavy wires are convenient for shuffling them around. Ventilate before any sparks or arcs. Occasionally a secondary battery can be returned to primary service due it’s status in an array might have been compromised by its neighbors. After discarding from the secondary bank usually they’re cores for exchange . Flooded lead acid golf cart batteries are still the best/cheapest for my service. I hesitate to discard cores that have all cells producing so a tertiary array of ‘problems’ for LED lighting will probably be set up in the future.
Maybe we can talk about NiFe, NiCd, NiH, and LiCoPO4 sometime 🙂
Maybe…Batteries are a very complicated topic. I already covered flooded and LiFPO on Off Grid Ham because they are the most common. I’m currently working on an article about SLA/AGM batteries which will probably be published next year. The rest are pretty weird and not often found in ham circles, so those topics will be deferred until there is more demand for information from the ham community.
As an antidote to the detail of my previous flooded lead acid comments………..getting together some “hand-me-down” batteries or emergency sign replacement batteries (takeouts), a solar panel, and some 12v led strings of lights can get one started. Some replenishible light and phone recharging power is a necessity. Then maybe a radio receiver or handheld CB, GSM, FRS, 2M ham radio. It’s just another increment to a good flooded acid, mobile car battery, or two good golf cart batteries to run a ham band transceiver. The Noontime Net on the west coast encourages mobile and lowpower checkins on 40m daily. My mobile battery in my M-37 was used to call for help in Alaska to an orbiting aircraft over Anchorage hundreds of miles away on HF (too far for VHF). Alaskan State Patrol found me and my two kids broken down and other hams brought me a tire from a hundred miles away. Since then, have helped others by CB on the desert and ocean while expeditioning.